Sample Environments SAXS/WAXS
Page Index
The beamline is capable of supporting a very diverse range of sample types and environments.
Beamline-Provided Sample Environments.
Here is information on sample environments that the beamline can supply for you. There are configurations for running samples in air, or in vacuum for ultimate signal:noise.
IN-AIR SAMPLES
Plates in Transmission.
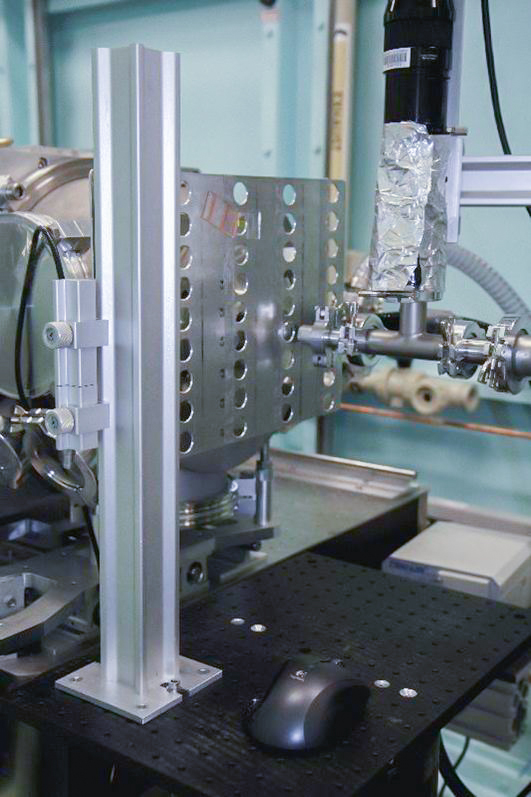
The simplest setup of them all - solid, powder, fibre or gel samples attach over or in holes in metal plates, and shot in transmission at ambient temperature. We have plates of various shapes and sizes:
the most commonly used format is based on the outer geometry of 96-well plates. Each laser-cut stainless steel plate contains 40 holes, each 5 x 5mm square in size, at 14 mm horizontal and vertical pitch. Each plate contains 5 rows and 8 columns of holes. Plates are either 0.7 or 2.0 mm thick to allow some choice of sample thickness when packing powder or gel samples . For ambient temperature measurement, two plates can be loaded into the beamline. The horizontal pitch is designed for mounting/sealing samples with 12mm tape (e.g. Kapton). Only use tape over your sample when needed to contain and/or seal it, otherwise keep tape away from where you will shoot the sample to keep background noise down. If you can fit your sample on this plate format, use it as because we have lots of these plates (so you can preload a lot of samples), and they also fit one of the temperature stages.
if you need holes larger than 5mm, there are several formats with 10 mm or 20 mm diameter holes at various pitches. There are only limited numbers of these plates, generally enough for one experiment at a time without running out unless you are running more than 100 or 200 samples. The larger plates cannot be temperature controlled.
Temperature Controlled Capillaries in Transmission.
These are used for temperature dependent studies, usually for fluid, gel and sometimes powder samples. Samples can be analysed in a batch, the temperature changed, and repeated automatically, so is often used to construct phase diagrams.
Standard X-ray capillaries can be run in batches of up to 70 at a time using two temperature controlled stages. Capillaries need to be standard length (80 mm) and up to 2.0 mm diameter. Each stage accommodates 35 capillaries at 5 mm spacing. Only the centre of the capillary is accessible to the beam. Each stage can also accommodate a small plate for solids or gels, 6 samples in each stage at a time.
Users need to supply capillaries, they are not provided by the beamline. When you buy capillaries, we recommend quartz capillaries for minimal background intensity. We recommend using 1.5mm diameter capillaries. Currently the stages are heated/cooled with water, allowing 10 - 80 ºC working range. We are in the process of upgrading this to a silicone oil bath to extend the temperature range from ~ -20 to 120 ºC.
Smaller diameter capillaries can be used with care but have two problems - they can move around more in the slots (making repeated acquisition quite difficult), and certainly below 1.0 mm diameter, scattering from the sides of the capillary (because of the horizontal width of the instrument’s background beam intensity) may compromise your data quality - expect problems below 0.8 mm diameter.
Quartz capillaries produce the best low-q SAXS background, better than borosilicate, “special” glass etc.
96 well plates (and related formats) in Transmission, with temperature control.
96 well plates can be shot in transmission mode in very high throughput mode. This is useful for diffraction samples where background scattering is not a problem, or as in many cases, peaks are identified/index without even background subtraction. It is routinely used for self-assembled liquid crystal materials, often robotically prepared and dispensed into 96-well plates. Well over 10,000 shots per day (actually around 20,000) shots per day can routinely be achieved.
Users need to supply wellplates for transmission work, they are not provided by the beamline.
The sample holder accommodates
2x 96-well plates
2x 40-well plates shown above
up to ~20 capillaries, which allow most of the full length of the capillary to be analysed (not just the centre like the 35-sample blocks).
One cover plate screws off/on for loading samples, which slide into a groove inside the holder. If you have not used this holder before, you may need to confirm the skirt on your plate format fits the slot that holds well plates (make sure it’s not too deep to fit).
Temperate control is by internally fan-forced air passing over the samples and through a fluid-controlled heat exchanger on the side of the stage. Due to limited airflow and heat exchanger area, the temperature range is limited to around 15 to 50 degrees. We may upgrade the fluid to use the silicone oil bath (late 2020) to extend the temperature range slightly.
Note - the temperature controlled stage has large Kapton windows, which add considerable background scattering. For well-plates that do not require temperature control, we have a simple windowless support frame that can hold two plates for ambient temperature analysis.
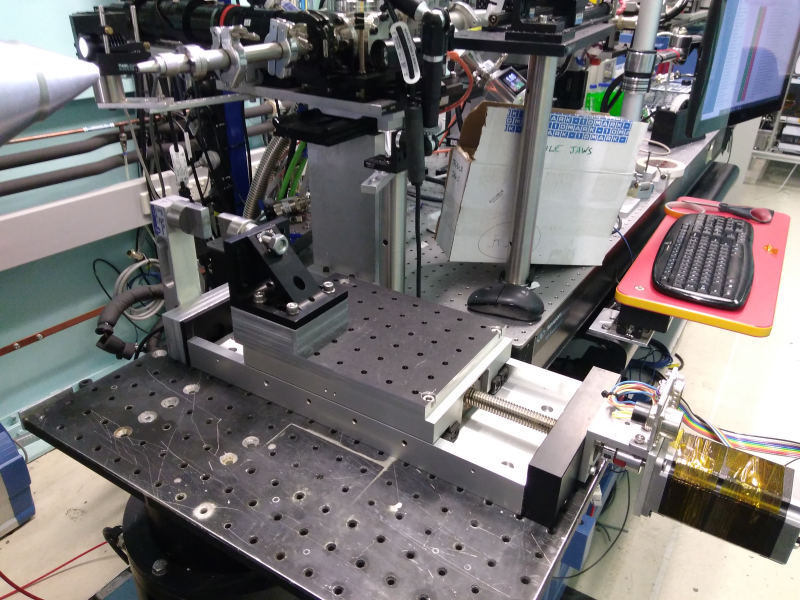
Mechanical Testing.
Samples can be shot in-situ under tensile or compressive strain. The stage is simply a high-capacity stepper motor driven linear stage, fitted with a loadcell and mounts for compression testing plattens or tensile grippers of various types. The sample is compressed or stretched by the stepper motor, whose rotation is measured by an encoder (at high load to check for motor step losses). There is no direct measurement of sample strain (e.g. extensometer) so sample deflection is calculated from the motor movement and by assuming no mechanical compliance in the stage (a reasonable approximation for the sorts of samples people generally measure).
The system has a well developed GUI for highly automated control of extension and SAXS data acquisition. The system is also capable of taking video still images of your sample for each SAXS exposure, and for samples that need wetting during mechanical testing, this control is built into the GUI control.
Loadcell capacity is 300 N. We also have a smaller one (~30 N) but has not previously been used.
There are a range of tensile grippers to suit various samples.
Linkam Systems.
We have a Linkam THMS600 temperature stage that can operate in air between -195 to 600 ºC, for samples measured in transmission. The cell can be flushed with various gases (e.g. dry air, N2, Ar, He ) if needed, which are standard gases we can supply and control at the beamline.
We also have a Linkam HFSX350 stage, which has a capillary attachment (for 1.5 to 1.7mm diameter capillaries). This can operate in air (and in future in vacuum) between -195 to 350 ºC.
Both units operate from our TS96 Controller, presently using Linkam software offline. Temperature control performance is superb, within 0.1 ºC, with virtually no overshoot/undershoot even at full ramp rates (e.g. 2.5 ºC/s heating or cooling).
Typically, only one sample at a time can be mounted.
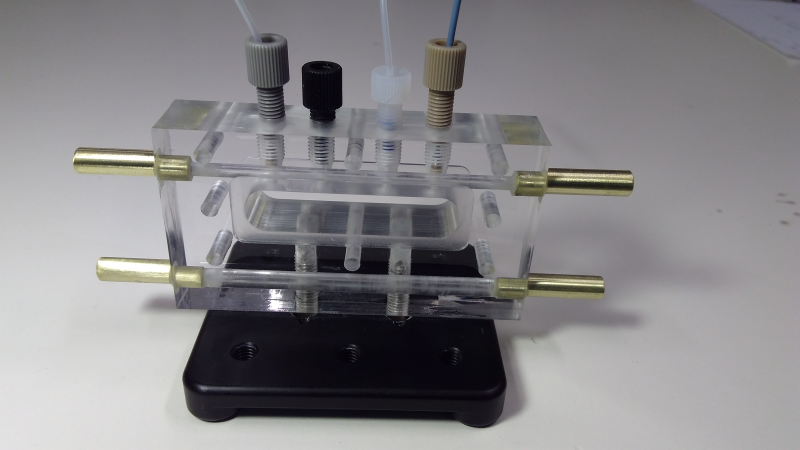
Grazing Incidence at Solid-Liquid Interface
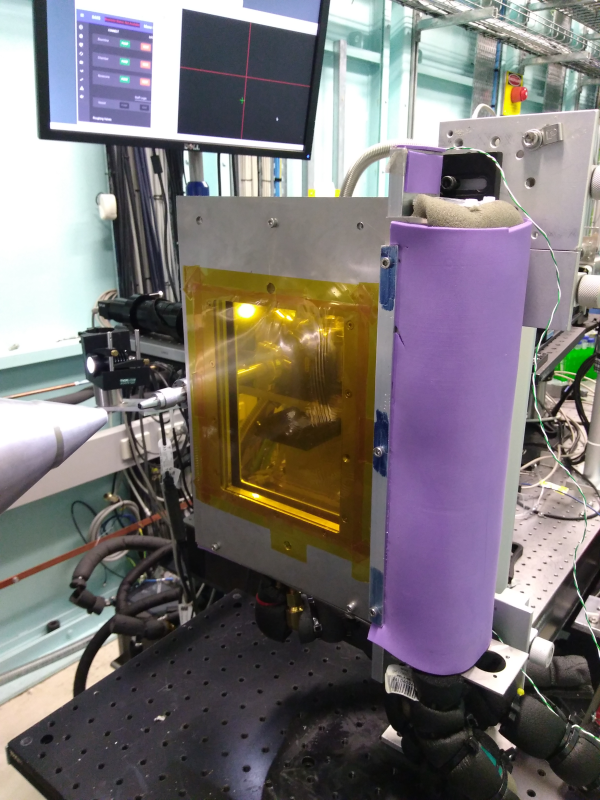
We have two simple cells (of similar design) that mount on in-air 2 or 3-circle grazing incidence stages (not shown) that allow grazing incidence GISAXS or GIWAXS on solid/liquid interfaces (or solid/controlled gas interfaces). Once cell (shown below) is made of PMMA (allowing electrochemistry) and a very similar cell has an aluminum body.
The from and back of the cell is closed by thin Kapton windows, clamped on by (but not in contact with the fluid) stainless steel plates (not shown). Temperature control is via internal fluid channels in the stage, nominally from ~10 to 70 degrees. Standard HPLC fittings (1/4-20 flanged fittings) allow connection of 1/16” HPLC type tubing, for inserting or flowing fluid through the cell, and/or inserting measurement electrodes for electrochemistry.
The sample cell is designed for samples up to 15 mm in the direction of the beam - samples are recommended to be 10 -15 mm long. The flat area of the cell is about 40 mm wide, so often several samples can be loaded and run side by side. The total fluid pathlength is 18 mm, so is used at 18 - 20 keV for adequate x-ray penetration - take account of this when considering your incident angles (i.e. critical angles are often ~ 0.05 º).
As show in the picture, the cell is usually mounted on a kinematic magnetic mount, so it can be removed for sample exchange and cleaning between runs, and repeatably replaced on the sample stages.
IN-VACUUM SAMPLES
If you need the incredible signal to noise obtainable by running in vacuum (and it IS lovely), and your samples at HV compatible (<1 x 10-5 mbar), then there are two main options for running in vacuum.
In-vacuum in transmission.
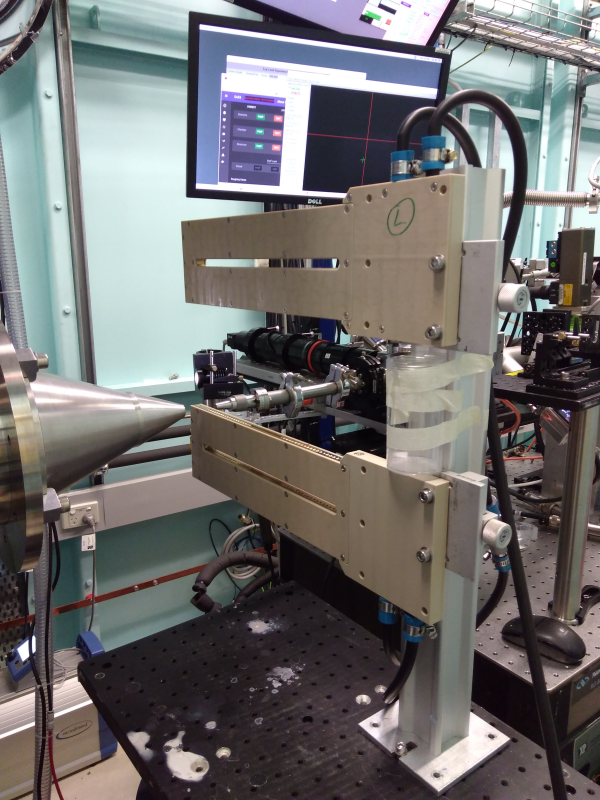
The sample vessel can be set up with two linear stages for transmission measurements of solids, fibres etc. Any simple plate-format can in principle be mounted, esp with a par of M6 x 25 mm pitch holes to attach to standard mechantronics brackets. The stage setup is shown below. Motor resolution of the horizontal and vertical stages is ~1 micron.
In-vacuum Grazing Incidence.
For ex-situ GISAXS or GIWAXS, we prefer you run in vacuum rather than in air. Not only do you get the exceptional signal to noise performance of vacuum analysis, its actually easier to set up because the scattered beam enters the detector chamber directly through the large gate valve, and there are no fiddly small vacuum windows to line up and can easily occlude the scattered beam.
Samples are mounted on a small in-vacuum hexapod. We recommend ~10 mm long (in beam direction) samples, roughly 10 mm wide. Shorter or longer samples can be run - smaller samples can be trickier to align (less rotational sensitivity, more footprint-overspill, less mechanical stability so can move during alignment) and long samples (> 20 mm) can be too rotationally sensitive, and will overhang the sample stage. Narrow samples (~5mm or less) can tend to move during alignment or analysis, but can be successfully run if required.
The ambient temperature stage is 20 mm (beam direction) by ~80 mm (lateral dimension) so multiple samples can be loaded in groups (e.g. 8 samples at a time).
Sample alignment is highly automated by a recursive python algorithm that runs height/pitch scans and automatically does this iteratively (if needed) and has proven highly reliable (i.e. convergent alignments) for samples within initial height/rotation tolerances. The alignment procedure uses a high resolution vertical beamstop slit, and the Pilatus SAXS detector, hence precision and accuracy can be high (~0.002 degrees) and X-ray dose during alignment is negligible (dose is typically 10 5 x lower during alignment than during single GISAXS/GIWAXS shot). Data acquisition is typically in “gapless” mode because most grazing experiments need full 2-dimensional data. Data acquisition after alignment is also fully automated. We are working on expanding the system to automatically align and acquire data not just from individual samples from multiple samples in groups.
We also have a Linkam temperature stage capable of operating in vacuum for grazing incidence scattering, with a temperature range of -195 to 350 ºC. Right now (May 2020) this is not operational in vacuum, but we are working on the plumbing modifications needed to make it functional.
User-Provided Sample Environments.
It is also common to bring your own sample environments and equipment. If you are planning this:
you must provide a description and details of equipment in your proposal. This allows its feasibility and safety to be assessed, during the beamtime allocation process. It is also critical information the beamline needs for scheduling experiments, so that beamtime is used efficiently and ensure you can make good use of your beamtime.
remember that a key aspect of many User-provided sample environments is electrical safety. Please ensure all electrical equipment has current testing and tagging prior to bringing it on site. We cannot provide testing and tagging for you.
take care with any other safety and reliability factors, such as pressure ratings/certification of components and systems, leak and spill containment, preventing of mechanical collision with beamline equipment.
the standard mounting equipment on the beamline uses M6 x 1.0 mm pitch screws, at 25.0mm spacing. Make sure you have the right hole spacings and/or adapter plates to mount your equipment on the sample table. We often use Newport X48 rail and carriers - part numbers are: rail X48-0.5, carriers either M-CXL48-80 or M-CXL48-50. For further information, see https://www.newport.com/f/48-mm-aluminum-four-sided-optical-rails
we have various pieces of equipment that you might use as part of your setup:
two syringe pumps, beamline controlled and automatable. Accommodates 1 mL to 50mL disposable syringes.
temperature sensor (K-type thermocouple, temperature displayed on monitors in cabin anf hutch, and temperature is logged automatically for each SAXS exposure)
sample table typically has a 300 x 450 mm metric breadboard (M6 holes at 25 x 25 mm pitch). This can be extended laterally either side (inboard or outboard) if more space is needed.
peristaltic pump, with single and 8-channel pump head (Masterflex)